
David F. Coker and Victor Batista
Department of Chemistry
Boston University
Large amounts of energy can be deposited in molecules by photoexciting them with lasers. If enough energy is deposited into an isolated molecule in this way, it may fly apart and dissociate into fragments (photodissociation). For excited molecules dissolved in liquid solutions or frozen into solid matrices, on the other hand, some of the excitation energy of the dissociating fragments can be dissipated as the fragments collide with their surroundings. This effect is known as solvent ``caging'' and it can lead to the excited molecule actually reforming soon after breaking apart. The recombined fragments generally still possess a lot of extra energy which may be partitioned in various ways in the reformed molecule. For example, the molecule may recombine with its electrons highly excited, or alternatively the excess energy of the recombined fragments may appear as rapid nuclear vibrational motion.
Our calculations provide a remarkably accurate interpretation of recent experiments on photodissociation and recombination in liquids and solids. Probing these processes of bond breaking and bond making and how they are influenced by solvent conditions provides a fundamental understanding of how chemical reactions can be controlled.
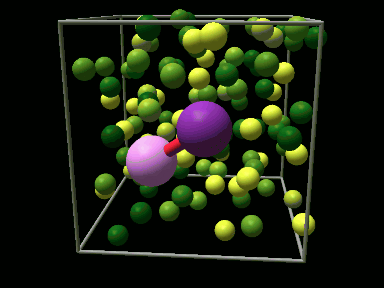
The first three video sequences show trajectories of a diatomic iodine molecular anion, I2¯, which starts out in its lowest energy electronic state at equilibrium with a surrounding liquid configuration of argon atoms. The I2¯ molecule next absorbs a photon of laser light and is photoexcited to a high energy electronic state in which the molecule begins to dissociate. Sequence 1 shows a trajectory where the surrounding liquid configuration is ineffective at caging the dissociating iodine fragments and they fly apart. In sequence 2 collisions with the liquid surroundings cause the dissociating iodine fragments to recombine and the I2¯ molecule reforms into a different excited electronic state in which it undergoes slow vibrational motions. Sequence 3 shows a trajectory in which the I2¯ recombines into its lowest energy electronic state leaving a large amount of excess energy in rapid vibrational motions of the reformed molecule.
The final video sequence shows photoexcitation of an I2¯ molecule frozen in a crystal of solid argon. The rigid nature of the crystal environment gives a strong solvent cage from which no dissociation fragments can escape. Thus recombination always takes place, with the molecule recombining into various different electronic states.
Sequence 1: Photoexcitation in liquid argon: Dissociation.
Sequence 2: Photoexcitation in liquid argon:
Dissociation followed by
electronically excited
recombination.
Sequence 3: Photoexcitation in liquid argon:
Dissociation followed by vibrationally excited recombination.
Sequence 4: Photodissociation and
recombination of I2¯ in solid
argon.
Back to the SCV Home Page